How Does Concentration Gradient Work?
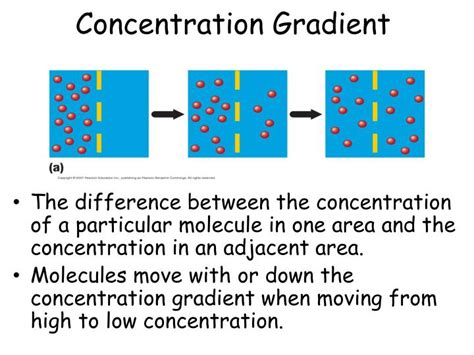
Concentration gradients are a fundamental concept in biology, chemistry, and physics, playing a crucial role in various natural and industrial processes. At its core, a concentration gradient refers to the gradual change in concentration of a substance across a particular distance or space. This phenomenon is essential for understanding how molecules move, interact, and distribute themselves within different environments.
To delve into the intricacies of concentration gradients, let’s explore the underlying principles and mechanisms that govern their behavior.
Definition and Basic Principles
A concentration gradient is established when there is a difference in the concentration of a substance between two points. This disparity creates a driving force that prompts the movement of molecules from the area of higher concentration to the area of lower concentration. The movement of molecules down their concentration gradient is a spontaneous process that tends to equalize the concentration throughout the system, a phenomenon known as diffusion.
The basic principle behind concentration gradients can be understood through Fick’s laws of diffusion. The first law states that the rate of diffusion of a substance is directly proportional to the concentration gradient. Essentially, the steeper the concentration gradient, the faster the rate of diffusion. The second law describes how the concentration changes over time, indicating that diffusion will continue until equilibrium is reached, where the concentration is uniform throughout the system.
Mechanisms of Action
Several mechanisms are involved in the establishment and maintenance of concentration gradients, including:
Passive Transport: This involves the movement of substances across cell membranes without the need for energy. Diffusion, osmosis, and facilitated diffusion (which uses transport proteins) are examples of passive transport mechanisms that contribute to concentration gradients.
Active Transport: In contrast to passive transport, active transport requires energy (usually in the form of ATP) to move substances against their concentration gradient. This process is crucial for maintaining concentration gradients that are not at equilibrium, enabling cells to accumulate or expel substances as needed.
Ion Channels and Pumps: Ion channels allow certain ions to move down their concentration gradient, while ion pumps use energy to transport ions against their concentration gradient. These mechanisms are vital for establishing and maintaining the resting potential of neurons and muscle cells, among other functions.
Biological and Industrial Applications
Concentration gradients have numerous applications and implications in both biological systems and industrial processes:
Cellular Respiration: The concentration gradient of protons across the mitochondrial inner membrane is crucial for the production of ATP during oxidative phosphorylation.
Nerve Conduction: The rapid changes in ion concentration gradients are essential for the generation and propagation of action potentials in neurons.
Industrial Separation Processes: Concentration gradients are utilized in various industrial applications, such as the separation of gases, the purification of water, and the concentration of solutes in solutions.
Pharmaceuticals: Understanding concentration gradients is vital for the development of drugs, including their absorption, distribution, metabolism, excretion, and toxicity (ADMET) properties.
Challenges and Future Directions
While concentration gradients are well understood at a fundamental level, there are ongoing challenges and areas of research, particularly in fields like biotechnology and nanotechnology. For instance, controlling and manipulating concentration gradients at the nanoscale could lead to breakthroughs in drug delivery systems and the development of novel materials.
Moreover, a deeper understanding of concentration gradients in complex systems, such as tissues and whole organisms, could provide insights into disease mechanisms and therapeutic strategies. The integration of computational models with experimental approaches is expected to play a significant role in advancing our knowledge of concentration gradients in these contexts.
Conclusion
In conclusion, concentration gradients are a cornerstone of biological and chemical processes, influencing a wide range of phenomena from the molecular to the organismal level. Their role in diffusion, active transport, and various industrial applications underscores their significance. As research continues to uncover the intricacies of concentration gradients, particularly in complex and dynamic systems, we can anticipate advancements in fields ranging from biomedicine to materials science.
What is the primary force driving the movement of molecules in a concentration gradient?
+The primary force driving the movement of molecules in a concentration gradient is the difference in concentration between two points, which prompts molecules to move from an area of higher concentration to an area of lower concentration until equilibrium is reached.
How do ion channels and pumps contribute to maintaining concentration gradients in cells?
+What role do concentration gradients play in industrial separation processes?
+Concentration gradients are utilized in various industrial separation processes, such as gas separation, water purification, and solute concentration. By controlling and manipulating concentration gradients, industries can efficiently separate components of mixtures based on their differences in concentration, which is critical for producing pure substances and materials.



