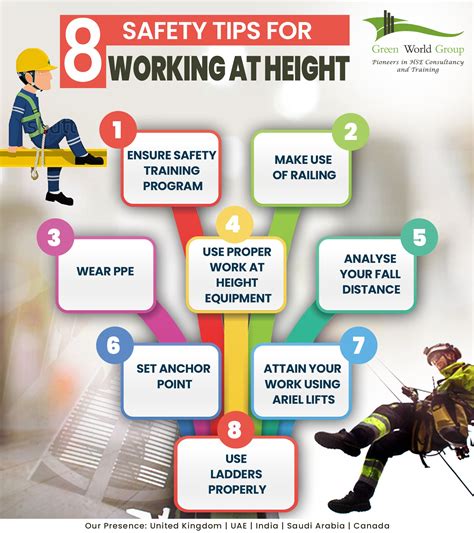
Lewis Structure Cabr2
Understanding the Lewis Structure of CaBr₂: A Comprehensive Guide
Calcium bromide (CaBr₂) is an ionic compound widely used in various applications, including drilling fluids, photography, and medicine. Its Lewis structure provides insights into its bonding, electron distribution, and chemical behavior. This article delves into the Lewis structure of CaBr₂, explaining its formation, properties, and significance in a clear, step-by-step manner.
What is a Lewis Structure?
A Lewis structure, also known as an electron dot diagram, represents the distribution of valence electrons in a molecule or ion. It uses dots to symbolize electrons and lines to indicate chemical bonds. For ionic compounds like CaBr₂, the Lewis structure highlights the transfer of electrons from a metal to a nonmetal, forming ions.Step-by-Step Construction of CaBr₂ Lewis Structure
- Identify the Elements and Their Valence Electrons: Calcium (Ca) is in Group 2 of the periodic table, so it has 2 valence electrons. Bromine (Br) is in Group 17, with 7 valence electrons. Since there are two bromine atoms in CaBr₂, the total valence electrons for bromine are 14.
- Determine the Type of Bonding: CaBr₂ is an ionic compound, meaning calcium donates its 2 valence electrons to each bromine atom, forming Ca²⁺ and 2Br⁻ ions.
- Write the Lewis Symbols for the Ions: - Calcium (Ca) loses 2 electrons to become Ca²⁺: [Ca]²⁺ - Bromine (Br) gains 1 electron to become Br⁻: [Br]⁻. Since there are two bromine ions, the structure is 2[Br]⁻.
- Combine the Ions to Form the Compound: The final Lewis structure of CaBr₂ is represented as [Ca]²⁺[Br]⁻[Br]⁻, indicating the ionic bond between calcium and bromine ions.
Key Characteristics of CaBr₂
Practical Applications of CaBr₂
- Oil and Gas Industry: Used in drilling fluids to control well pressure and prevent formation damage.
- Photography: Acts as a component in photographic emulsions.
- Medicine: Utilized in certain medical treatments due to its bromide content.
Comparative Analysis: CaBr₂ vs. Other Ionic Compounds
| Compound | Formula | Melting Point (°C) | Solubility in Water |
|---|---|---|---|
| Calcium Bromide | CaBr₂ | 730 | Highly Soluble |
| Sodium Chloride | NaCl | 801 | Highly Soluble |
| Magnesium Sulfate | MgSO₄ | 1,124 | Highly Soluble |
Frequently Asked Questions (FAQ)
Why is CaBr₂ considered an ionic compound?
+CaBr₂ is ionic because calcium (a metal) transfers its valence electrons to bromine (a nonmetal), forming positively charged Ca²⁺ and negatively charged Br⁻ ions held together by electrostatic forces.
How does the Lewis structure of CaBr₂ differ from that of a covalent compound?
+Unlike covalent compounds, which share electrons to form bonds, the Lewis structure of CaBr₂ shows electron transfer, resulting in separate ions rather than a shared electron pair.
What is the role of CaBr₂ in drilling fluids?
+CaBr₂ increases the density of drilling fluids, helping to control well pressure, stabilize the borehole, and prevent blowouts during oil and gas extraction.
Can CaBr₂ conduct electricity in its solid and liquid states?
+In its solid state, CaBr₂ cannot conduct electricity due to fixed ions. However, when melted or dissolved in water, the free movement of ions allows it to conduct electricity.
Understanding the Lewis structure of CaBr₂ not only clarifies its ionic nature but also provides a foundation for predicting its behavior in chemical reactions and practical applications. Its simplicity and versatility make it a valuable compound in both industrial and scientific contexts.